FDA clearance for a diagnostic imaging machine indicates that the device has been deemed safe and effective for its intended use by the Food and Drug Administration (FDA) in the United States. This clearance process involves thorough evaluation of the device’s design, performance, and manufacturing processes to ensure that it meets regulatory standards for quality, safety, and efficacy. Here’s the latest devices that have received FDA clearance.
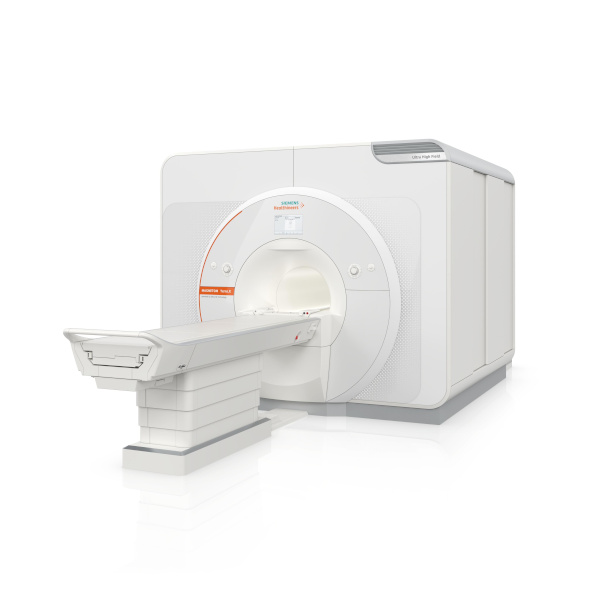
The Magnetom Terra.X: MRI System
The Magnetom Terra.X, a new 7T MRI system, has received 510(k) clearance from the FDA. Manufactured by Siemens Healthineers, it’s a second-generation successor to the Magnetom Terra and offers several enhancements for 7T imaging. Key features include an eight-channel parallel transmit architecture for clinical use, deep learning image reconstruction optimized for 7T, improved diffusion imaging with a high-performance gradient system, and accelerated image acquisition enabling high-resolution brain and knee exams in under 20 minutes. Siemens Healthineers sees this as a significant step in providing better patient care, particularly in neurological and knee imaging. Additionally, the FDA clearance allows existing Magnetom Terra systems to be upgraded to the Magnetom Terra.X.
SyMRI 3D for Brain Imaging
SyntheticMR has announced that its latest imaging solution, SyMRI 3D, has received FDA 510(k) clearance for clinical use in the United States. This clearance marks a significant advancement in quantitative MRI technology, offering exceptional resolution and accuracy in brain imaging. SyMRI 3D enables precise volumetric estimations of brain regions, known as parcellation, providing clinicians with deeper insights into brain structure and function. The enhanced resolution facilitates comprehensive lesion analysis, leading to more accurate medical condition assessments. This clearance empowers physicians to make more informed decisions in diagnosis and treatment planning, ultimately improving patient outcomes. SyntheticMR reaffirms its dedication to advancing medical imaging technology and providing innovative tools to enhance patient care through this milestone.
nCommand Lite for Remote Scanning
GE Healthcare has highlighted the FDA clearance of a solution by Ionic Health that enables technologists to remotely supervise patient scans. The system, called “nCommand Lite,” has been tested in Brazil for three years and is vendor-agnostic, allowing remote supervision across MRI, CT, and PET modalities. GE has secured exclusive distribution rights for nCommand in the U.S., aiming to address ongoing workforce shortages in healthcare. Rekha Ranganathan, GE’s chief digital officer for imaging, emphasized the company’s commitment to remote operations and increasing patient access to expert technologists. The system facilitates not only scanning supervision but also training, procedure assessment, and scanning parameter management. GE’s announcement coincides with growing interest in remote scanning, with the American College of Radiology advocating for permanent remote supervision of diagnostic tests. However, technologists have expressed reservations about managing imaging remotely, according to recent survey data from the American Society of Radiologic Technologists.
Sources:
Itnonline.com
Radiologybusiness.com
diagnosticimaging.com
openai.com



